Understanding Borate


The use of borates in pools has gained momentum over the past few years by service techs and homeowners alike. As its acceptance grows, the data and anecdotes of borate benefits are mounting, pointing toward a very bright future for boron-based treatment products.
By Terry Arko
This article is dedicated to the memory of Robert W. Lowry, long-time, instructor, author and chemist, and co-founder of the Pool Chemistry Training Institute.
Borates are gaining popularity and are now widely recommended for pools, and for good reasons: For starters, adding borate is a one-time chemical affair. It does not decompose, get used up or expire.
Borate is only lowered by water loss – splash out, drag out, leaks, filter backwashing, or draining. You add it once, and unless you are losing a lot of water, you don’t need to add it again until you drain the pool and refill it. But why use it in the first place?
The benefits of using borate are truly impressive:
- Keeps the pH of the water from going up (pH buffer against rises in pH)
- Helps prevent algae (it is an algaestat, not an algaecide)
- Lowers chlorine demand (preventing algae lowers the need for chlorine)
- Helps protect HOCl (hypochlorous acid – the killing form of free chlorine) from UV destruction
- Water feels softer and silkier
- Water looks clearer and sparkles
Except for the last two points, these are not just manufacturer or sales claims but proven benefits when the concentration of borate in the water is 50 ppm. Stabilizing pH helps with balance-related concerns, and by reducing chlorine demand, it helps mitigate today’s skyrocketing costs.
There is no published evidence in physical science for a mechanism of enhanced blue color or sparkle or for a silkier or softer feel to water. However, these anecdotal claims still persist in manufacturer’s literature, service tech observations and pool owner reports.
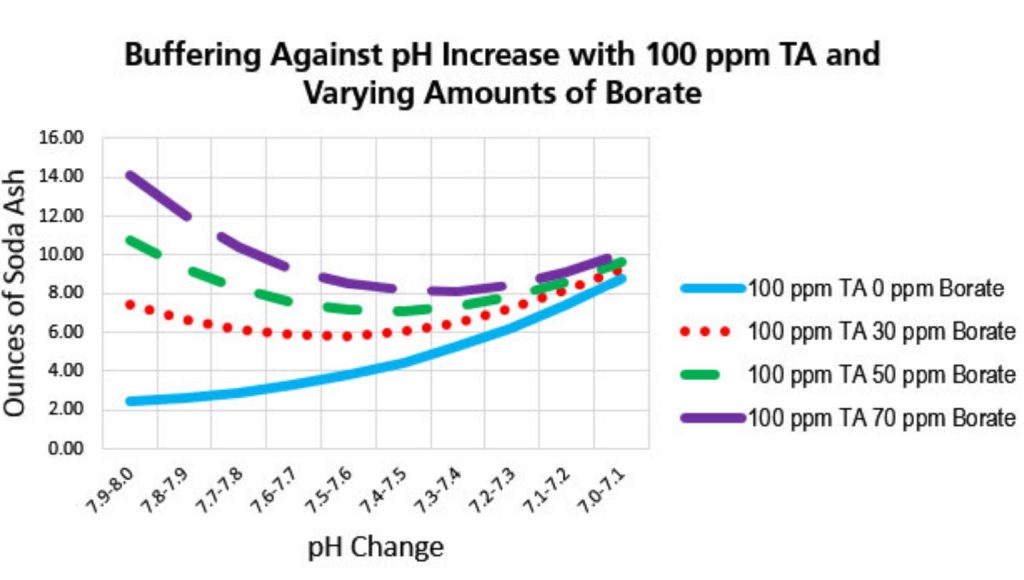
Borate as a pH buffer: Buffering is when the water resists changes in pH when acid or base are added to it. When we think of pH buffering, most of us have been taught to think in terms of Total Alkalinity (TA.) The measure of bicarbonates (HCO –) and carbonates (CO –2). Most of the TA in pool water is made up of bicarbonate.
Adding borate to the water prevents the pH from rising so borate can be rightfully considered a pH buffer too. Keeping TA at 90 ppm, cyanuric acid at 30-50 ppm and borate at 50 ppm, makes an ideal overall buffering system against up or down pH changes.
SALT SYSTEM ASSIST
If the pool has a Saltwater Chlorine Generator (SWG), borate is especially helpful because in the process of making chlorine from salt, SWGs produce a high pH. This requires acid to be added to lower the pH but acid also lowers alkalinity. The result is high pH and low alkalinity. This requires addition of sodium bicarb to raise alkalinity.
Pools equipped with SWG or ECG (electronic chlorine generator) will benefit from a level of borate of 50 to 70 ppm, however, the US EPA and NSF do not recommend levels more than 50 ppm.
Borate is a buffer against pH increase so this means less calcium carbonate scaling on the hydrogen gas (H2) generation plate (negative electrode) of the SWG. That should be its only side effect and it is a benefit. Also, keeping 50 ppm of borate lowers the recommendation that free chlorine should be 7.5% of CYA to 5% of CYA. This is a minimum of 33% less chlorine.
Using borate as a buffer with an SWG slows the rate of pH rise. The pH may still rise, but not as dramatically. So, you will need to add acid less frequently. Bicarb will not be needed as often either. Ideal borate level with SWG is 50-70 ppm borate, however, the EPA maximum is 50 ppm with a level of concern of 100 times that.
BORATE AS AN ALGAESTAT
Many articles claim that borate is an algaecide and that borate works as an algaecide by removing CO2 from the water. Algae require CO2 to carry out photosynthesis so removing CO2 would kill algae. Logical but wrong. Algae are carbon-based life forms. The fact is that algae can get plenty of carbon or carbon dioxide (CO2) from the carbonates (CO –2) and bicarbonates (HCO –) in the water (TA).
Borate does not remove any CO2 from the water and even if it did, the next time soda ash or sodium bicarb is added, CO2 is added. In addition, CO2 from our atmosphere is continuously dissolving into the water because CO2 in the water is at equilibrium with CO2 in the atmosphere and that would be enough to support algal growth.
How does borate prevent algae? It does it by disrupting cell-wall development, metabolism, and cell division. So, borate is good at reducing chlorine demand because you are preventing algae. Some manufacturers claim 50% reduction in chlorine consumption.
Boric acid, formula B(OH)3 or H3BO3, is technically a Lewis acid. It does not release a hydrogen ion (H+) like most acids, but it can acquire an OH– from the water according to this equation:
B(OH)3 + H2O ↔ B(OH)4- + H+
Borate Products
We recommend using boric acid because the pH adjustment is minor and the overall cost is the least expensive. However, finding boric acid in your area may be difficult.
Boric Acid: The chemical formula is B(OH)3 or H3BO3, same thing. Boric acid is sold at home centers, hardware and gardening stores. It is often sold in nurseries and garden stores as a bug killer.
Adding 76 oz or 4.75 lbs of boric acid per 10,000 gallons of water will provide 10 ppm of borate.
Boric acid is a weak acid and has a pH of 3.8-4.8. It will not lower pool water pH by much. Most of the time the pH drop is only about 0.2 for a 50 ppm dose. Therefore, you can add boric acid without having to add muriatic acid like when adding Borax or sodium tetraborate pentahydrate.
Also, if you have metals in the water or have had metal staining, boric acid will not cause additional staining, deposits, or precipitation. No problem with high pH and alkalinity either. In fact, you just add it and in about 12 hours or so, you test pH and alkalinity and adjust them if necessary.
Borax (not Boraxo): The chemical name is sodium tetraborate decahydrate, formula Na2B4O7•10H2O. This is probably best known as 20 Mule Team Borax™. It is often found in grocery stores in the laundry aisle. Boraxo™ is soap or laundry detergent. You don’t want this.
Adding 118 oz or 7.4 lbs of Borax per 10,000 gallons of pool water will provide 10 ppm borate.
Borax has a pH of about 9.2 and it will raise the pH of the pool water when added. This has two potential problems.
First, if there are any metals in the water (copper, iron or manganese) there is a possibility of causing a stain. Metals may be dissolved in the water and may be at the saturation point for that pH, alkalinity and hardness. When the pH is raised by adding Borax the metals may precipitate out causing a stain.
So, if you have had problems with metal stains in the past or if you are using well water, you should consider testing the water for metals and then using a metal remover, stain inhibitor chemical (called sequestering agents) or using another borate-containing product such as boric acid or a borate pH neutralized product instead of Borax (see below).
Second, to offset the high pH of the Borax you will need to add muriatic acid (31.45% HCl). The amount needed is 3 gallons or 10 boxes of Borax or 2 fl oz of muriatic acid for each 1 oz of Borax added.
In addition, before adding the Borax, the alkalinity should be about 80 ppm and the pH should be about 7.4 or less. The TA must be lower than 140 ppm and the Hardness must be less than 350 ppm before adding Borax. Adjusting these levels may or may not be needed. Test the water to be sure and make the adjustments.
Cost is not the only consideration in choosing a borate product. Availability, ease of use, convenience, muriatic acid use, storage, handling, personal safety, time and effort are all considerations.
Boric Acid or Sodium Tetraborate Pentahydrate
If you use sodium tetraborate pentahydrate (let’s just use tetraborate) you will increase pH and TA in addition to raising borate in the water.
This requires adding a large amount of acid to lower TA back to a target of 90 ppm which will lower pH much lower than 7.0. Then you need to aerate and create turbulence and splashing to raise only the pH. If you add soda ash to raise the pH back up, you will also raise alkalinity which makes TA too high.
If you use boric acid to increase borate in the water, it will only lower pH by 0.2 and have no effect on TA.
Testing for Borate
You will need to buy some borate test strips. Over time with water loss from backwashing, filter cleaning, splash out, drag out and other water loss, (but not evaporation), you will need to bring the level back up to 50 ppm or more.
The only way to do this is to know the borate level in the water. Borate test strips are made by LaMotte, ITS, Hach and Taylor.
Check the borate level about once a month. Whenever the level gets near 30 ppm, you should add sufficient borate to bring it back to 50-60 ppm. It is no problem and there are some benefits from higher levels but never more than 70 ppm.
Borate Toxicity
Several common fruits and vegetables typically contain 160-300 ppm boron. Sea water contains about 4.5 ppm boron and some saline lakes contain above 300 ppm boron.
The “Acute LD50” is the amount of a chemical necessary for a lethal dose of 50% of the test subjects. It is usually expressed as grams/kilogram (g/kg). Acute oral LD50 for boric acid in laboratory animals is in the range 2.5-5 g/kg; about the same as table salt (NaCl: LD50 3-4 g/kg).
For an individual weighing 70 kg (150 lbs), this would indicate more than 150 g or 5.3 oz of pure boric acid for lethal effect. From a study of fewer individuals, dogs may be about 4-fold more sensitive. From accidental poisonings in humans, minimum oral lethal doses of boric acid have been estimated to be in the range of 5-20 g for adults, 3-6 g for children.
To obtain a 5 g dose of boric acid from a swimming pool at 50 ppm boron it would be necessary to swallow 20 liters (about 5.25 gallons) of pool water.
Dogs and cats should not drink pool water because it contains many chemicals that are not good for our pets (or us!.) Provide fresh water outside and change it regularly. It is fairly easy to teach pets not to drink from the pool. A good tip is to put a chair on the top step of the pool access. Dogs usually stand on the top step to drink. Blocking access helps.
In the 2008 Tolerance Reassessment Eligibility Decision (TRED), the U.S. EPA (2008b) selected a no observable adverse effect level (NOAEL) of 8.8 mg/kg-day (as boron) from a chronic toxicity study in dogs in order to assess the margin of exposures for various residential uses of boron (including its use in swimming pools).
Similarly in 2015, the U.S. EPA calculated a margin of exposure of 160 for children ages 6 to IO as compared to the LOC (Note: I don’t know what LOC is. Is it defined anywhere else?) of 100, which again suggests that a higher application rate may be acceptable. Nevertheless, both U.S. EPA assessments (2008b; 2015) indicate that an application rate resulting in a concentration of 35 mg/L (as boron) is currently authorized under FIFRA.
Assessment of boron under NSF/ANSI 50 also determined that the currently recommended application rates of boron (resulting in concentrations of 35-50 mg/L) did not pose a substantial risk to human health and this exposure has an LOC of 150 so you could use 1.5 times that amount with a margin of safety of 100 times.
Our recommendation stands at 50 ppm borate for pools and spas.
Terry Arko is Product Training and Content Manager at Hasa Pool Inc.
Opening image by Magnetix | Shutterstock