The Perils of Ocean Acidification


The data is clear, the vast ocean of planet earth is becoming more acidic as atmospheric carbon dioxide levels increase. But what does that change really mean? Is ocean acidification a problem now, or in the future? Or is it just a point of passing interest as nature adapts to changing conditions?
By Eric Herman
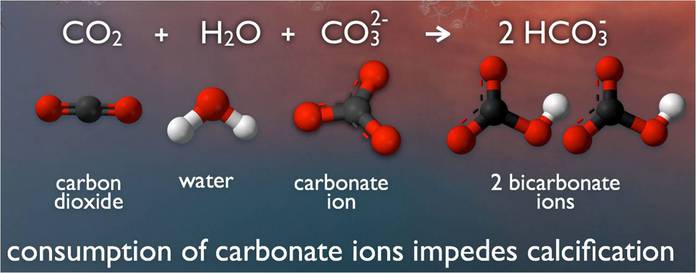
Ocean water on our planet is becoming more acidic. In concept, the chemistry is not terribly complex. When carbon dioxide (CO2) is absorbed by ocean water, chemical reactions occur that reduce the water’s, carbonate ion concentration, thus lowering pH. These chemical reactions are termed “ocean acidification” or “OA” for short.
As the CO2 in the atmosphere rises, the oceans absorb a lot of it. According to data maintained by a host of organizations studying the issue, such as National Oceanic and Atmospheric Administration, oceans not only generate 50% of the oxygen in our atmosphere, they absorb 25% of all carbon dioxide emissions while capturing 90% of the excess heat generated by these emissions.
It is not just ‘the lungs of the planet’ as some describe the ocean, but also the planet’s largest “carbon sink”.
In this way, the earth’s ocean is central to reducing global greenhouse gas emissions and stabilizing the climate. That’s a very important role, no doubt; but, it does come at what could be a catastrophic price – and it all deals with subjects that are near and dear to watershaping professionals, i.e., pH and calcium.
In marine environments, minerals containing calcium carbonate are the building blocks for the skeletons and shells of many marine organisms. In areas where most ocean life exists, the water is supersaturated with calcium carbonate minerals.
his means there is abundant material for calcifying organisms to build their skeletons and shells. However, continued ocean acidification is causing many parts of the ocean to become undersaturated with these minerals, which is likely to affect the ability of some organisms to produce and maintain their shells.
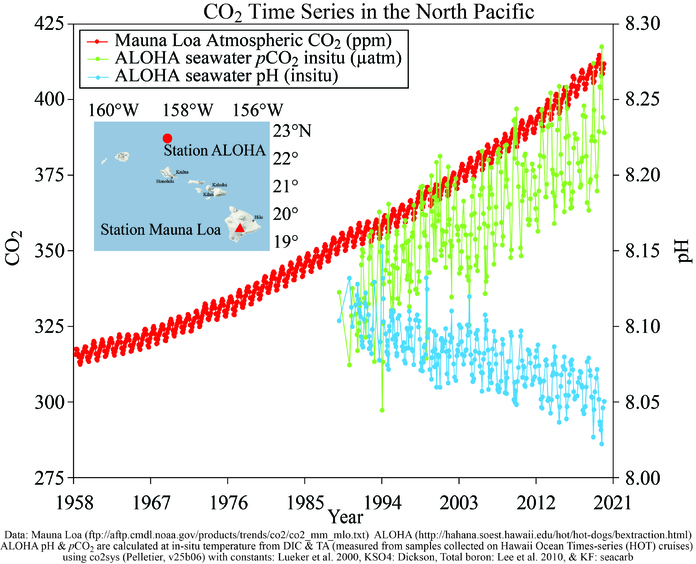
Since the beginning of the Industrial Revolution, the pH of surface ocean waters has fallen by 0.1 pH units. Doesn’t sound like much? Think again. Because the pH scale is logarithmic, that change represents a 30% increase in acidity.
Estimates of future carbon dioxide levels indicate that by the end of this century, the surface waters of the ocean could have acidity levels nearly 150% higher than today, resulting in a pH that the oceans haven’t experienced for more than 20 million years.
LIFE IMPACT
Alarmist? Perhaps. Still there is little question that OA is expected to impact a spectrum of marine species. On one hand, photosynthetic algae and seagrasses may actually benefit from higher CO2 conditions in the ocean, as they require CO2 to live just like terrestrial plants. By contrast, studies have shown that lower environmental calcium carbonate saturation can have a devastating effect on many calcifying species, including oysters, clams, sea urchins, shallow water corals, deep sea corals, and calcareous plankton.
Among the many dire ramifications for humans, the reduction in dissolved calcium carbonate could dramatically impact ocean food production. Today, more than a billion people worldwide rely on food from the ocean as their primary source of protein. As a result, jobs and food security in the U.S. and around the world depend on healthy fish and shellfish populations.
OA threatens that food source and entire industry.
We are currently watching this decline play out in real time. In recent years, there have been near total failures of developing oysters in both aquaculture facilities and natural ecosystems on the U.S. West Coast.
Although more research is needed, low pH is likely a factor contributing to oyster reproductive failure along with pathogen increases and low dissolved oxygen. Such concerns are prompting accelerated research on OA and its impacts on individual species as well as overall biodiversity.
Increasing OA has been shown to significantly reduce the ability of reef-building corals to produce their skeletons. Coral biologists have recently reported that OA could compromise the successful fertilization, larval settlement and survivorship of Elkhorn coral, an endangered species.
BAD DIRECTION
Other research indicates that, by the turn of the century, coral reefs might erode faster than they can be rebuilt. This could compromise the long-term viability of these ecosystems and perhaps impact the estimated one million species that depend on coral reef habitat.
All in all, it’s fair to conclude that OA is an emerging global concern.
Because sustained efforts to monitor ocean pH worldwide are only beginning, it is currently impossible to predict exactly how OA impacts will ripple throughout the marine food chain, and ultimately affect the overall structure of marine ecosystems. With the pace of OA accelerating, scientists, resource managers, and policymakers would do well to recognize the urgent need to strengthen the science as a basis for sound decision-making and action.
Opening image by Richard Whitcomb | Shutterstock