Elemental Finesse

{Multithumb}
Ever since people decided to contain and control water for recreational and decorative uses, there have been competing ideas about how to treat it so that it remains safe for human contact. That environment has become even more intense in recent years, as questions and concerns have arisen about the continuing use of traditional chlorine chemistry to get this important job done.
Today, for example, we hear a lot about “natural pools” – systems using plant material to absorb the nutrients that feed algae and bacteria. There’s also ozone technology, which needs to be combined with stabilized halogen to treat water effectively. Then there are copper/silver ionization systems and their cousins, the saltwater chlorinators, which have taken root and gained support in many quarters.
My career working with alternative sanitizers began a few decades ago. About three years ago, my firm – Fluid Logics of Upland, Calif. – entered this arena with the thought in mind that the watershaping industry needed to take a broader view of the last of those alternatives, digging back through the 100-year history of electrolytic chemical generation and expanding the capacity of these systems to oxidize organic compounds and sanitize the water.
For several years before then, I had sold and installed saltwater chlorination systems in the pool/spa industry. I did my homework and came to believe that the technology could be improved to a point where we could generate greater levels of chlorine than existing systems could – and might even be able to do it without needing to add so much salt to the water.
When I had this epiphany, I recognized that the basic technology could be tailored to a variety of other applications in this and other industries. That’s when I set out to develop my own version of electrolytic water treatment and make it an even better alternative to traditional approaches.
CONSTANT CONTAMINATION
Although technologies have changed and in some cases have even come and gone through the years, the fundamental challenge of treating water remains the same: When it comes into contact with humans, it must be free of bacteria and of the organic compounds that engender their growth. This is why chlorine has been such a success in swimming pools and spas: It does both jobs quite effectively – so much so that when it comes to bodies of water used by large numbers of people, it’s tough to find a better option.
In recent times, however, chlorine has suffered in the realm of public opinion and many consumers have sought ways to avoid use of chlorine products that have to be manufactured, packaged, transported, stored and added to water. That’s all a hassle and can be hazardous, and so, whatever its merits, chlorine is becoming distinctly unpopular.
Ironically, several of the options to basic chlorination still use chlorine – particularly saltwater chlorination systems, in which sodium chloride (common table salt) is transformed into sodium hypochlorite (bleach) to treat the water. My ambition was to take this simple electrolytic concept and put it on steroids so the technology would become even more effective not only with smallish pools and spas, but also in other applications involving far larger volumes or bodies of water.
| When we began developing systems, we found that, by working with the composition of the coatings on our electrolytic cells, we could manipulate their chemical output and make them suitable for use in a wide range of applications including (as shown here) oil production, industrial research and winemaking, among many others. |
The key to this expansion of applications flowed from recognizing that, with electrolysis, chlorine generation is only part of the picture. Indeed, I quickly came to appreciate the fact that, using this technology, we could do far more than generate chlorine by using salt dissolved in water – and that many other constituents in water, treated electrolytically, can produce a range of compounds that also can be useful in water treatment.
These added compounds – ozone, nascent oxygen, hydrogen peroxide among them – each has its own characteristics and can be used quite effectively, depending on the application. The crux of the matter is that these entities stay in solution for varying lengths of time, giving us the opportunity to work with those different contact-time characteristics to target various harmful pathogens and organic compounds before the beneficial compounds themselves are destroyed by sunlight or decomposition.
Take ozone as a familiar example: Any watershaper who has worked with it to any extent knows that it’s a powerful sanitizer and oxidizer, but that it remains in solution in water for only a few seconds to a few minutes and therefore must be constantly generated or used in conjunction with another chemical (typically chlorine as a residual barrier) to extend the periods during which water is safely treated.
Less familiar is nascent oxygen (also known as the “hydroxyl radical”), which is similar to ozone and is, in fact, the component that attaches to O2 (oxygen) to make O3 (ozone). Nascent oxygen exists in water for only fractions of a second. There’s also hydrogen peroxide, which may be familiar to those who’ve thoroughly explored the realm of chlorine alternatives: It, too, is a powerful oxidizer that works by way of adding hydroxyl radicals to the water.
Using these possibilities, we’ve been able to push electrolytic water-treatment technology well beyond small pools and spas to the point where we now are working with applications in treatment of stormwater, sewage, gray water, marine-animal exhibits, large-scale ponds and streams, lakes, big waterparks and a host of industrial applications.
EXPANDING THE DISCUSSION
The big difference between existing pool/spa saltwater chlorinators and the technology we’re pursuing is that the amount of chemicals generated relative to the amount of power required to create the electrolytic reaction has been altered dramatically: In other words, these new system offer much more bang for the buck.
We started down this path of examining chlorine generation because we saw untapped potential in the technology and figured we could advance the cause of delivering all of the benefits of chlorine water treatment while minimizing the downsides involved in its byproducts, the cost of production and the hazards and costs of producing, storing, transporting and adding it to water.
As our research continued, we came across an odd fact: Few people think of it this way, but ocean water is, in fact, chlorinated. Seven-tenths of the earth’s surface is covered with water that contains sodium chloride – water that contains plants that survive by photosynthesis, a process that generates infinitesimally small amounts of electricity.
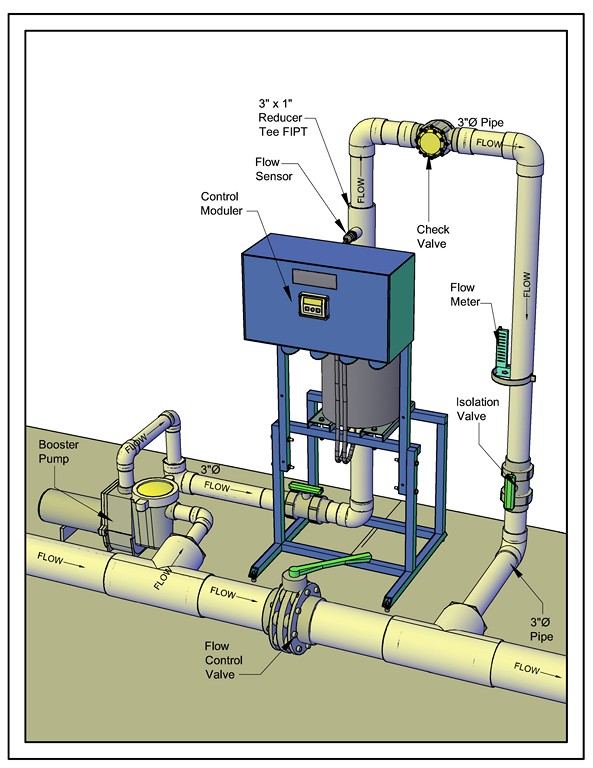
| No matter the industry or application or volume of water, setting up one of these systems involves pretty much the same configuration of pipes and fittings that direct the flow of a defined quantity of water to and through the electrolytic cell. |
This electrolytic potential does, in point of fact, generate chlorine from chloride ions that play a part in the earth’s natural system of biological checks and balances. Add to that the fact that sodium chloride itself has antiseptic qualities and that dissolved oxygen is always present in ocean water through the churning action of wind, waves and surf and you have a system in which a vast volume of water carries and manages incalculable volumes of organic compounds. We recognized that, in a very real sense, we could use electrolytic technology to mimic these natural processes and therefore treat bodies of water far larger than anyone would have imagined.
On the most basic level, there are three primary elements in these systems: the anodes, the chamber in which the reaction takes place and the power supply. In system development, we spent a good bit of time evaluating these key components in electrolytic systems and how they interrelate.
We learned that anode technology has come a long way since the first systems were developed in the 19th Century. Without going into too much detail, manufacturers have experimented with a variety of materials, including titanium, iridium, platinum, copper and silver (to name a few), working to find anode coatings that efficiently transfer electrical energy to water while withstanding the intense electrolytic and chemical environment. Long story short, our systems use a proprietary coating that reflects the latest developments in anode science.
We can vary the ingredients of the coatings to perform different tasks, such as producing different chemicals in conjunction with other electrolytes or lower levels of certain electrolytes. Lower salt levels, for example, actually create a more aggressive environment for the cell coatings, so in some circumstances we must make the cells more robust.
The chambers that contains these anodes – the places where the electrolytic reactions actually happen – have also undergone extensive development related to size, shape and hydraulic cycling, and there’s also been a large volume of research on the sizes of anode surfaces relative to water flow. Again, we took the best of that recent research and applied those insights to our systems.
A LEAP FORWARD
Where the big advancements have come recently (and where we saw our greatest opportunity to advance the technology) is in the power supply portion of the equation.
The relationship between the anode and the power that feeds it requires extremely precise control to maximize system efficiency and output. Our work focused primarily on using integrated-circuit technology to condition the power and enable us to produce dramatically increased chemical outputs while keeping energy consumption to a minimum, no matter the power source.
The electronics involved here are fairly sophisticated. Without revealing too much about our approach, suffice it to say we condition alternating-current (AC) power to deliver direct current (DC) to the anode in a way that generates much less heat and instead transfers that energy to the anode. The system runs cooler, consumes less power and delivers increased output.
The upshot of all this is that these systems can operate across a wider range of applications in terms of vessel size and nutrient load. Moreover, because the volumes of the chemicals generated by these systems can be so much greater, the way these chemicals work together becomes more important. In most bodies of water, in fact, there are multiple available constituents that will, when subject to electrolysis, produce oxidizers and sanitizers.
Our systems, for example, can be modified to generate elevated levels of nascent oxygen to increase sanitizing and oxidizing effects. In most cases, in fact, that output reaches such high levels that it’s not even necessary to add salt to generate chlorine: Instead, we simply use the water’s natural saline constituency to do the job.
| By rethinking a technology that’s been around for more than a century, we’ve broken the volume barrier that had previously limited the potential of saltwater chlorine generation and have developed systems that deliver high levels of desired chemicals – and do so in an energy-efficient (and therefore cost-effective) manner. |
Water that’s been treated at some point with sodium hypochlorite, for instance, will always contain some residual level of salt, so given the system’s efficiency, it’s unlikely we’ll need to add more than a bit of salt to and existing watershape system to generate a chlorine residual.
That’s significant with pool/spa applications, but we also observed that it might have even larger implications for other bodies of water in which low sanitizer concentrations are desirable, as with lakes or large ponds. By combining the effect of chlorine generation (or even hydrogen peroxide generation) with elevated levels of nascent oxygen, we’re able to open up the technology to applications where on-site chemical generation has never been considered before.
Doubling back to the technology itself, materials science has taught us that by using different compounds on the anodes, we can either encourage or inhibit the production of specific chemicals and, in effect, can fine-tune our systems to specific applications. In effect, we can create systems that generate only nascent oxygen and no chlorine. That’s not something that means much to the pool/spa industry, but it certainly has relevance for those in the business of maintaining exhibits with large marine animals or of enhancing the performance of wetlands-treatment systems.
A FAIR START
We’re just three years into the development of our systems and so far have spent the bulk of our time simply finding ways to tune our systems to meet very specific sets of demands. We’ve found a huge interest in the technology within the petroleum industry, for example, where companies use water reinjection to fill subsurface voids left behind by removal of raw petroleum or to enhance the output of wells. For reasons I won’t discuss here, this water must be treated in very specific ways we’ve been able to master.
The treatment requirements of that application are vastly different from those require to prepare water for direct human contact, but the principles remain the same – and we believe we’re positioned to use our systems to address a variety of current and future needs with great accuracy and efficiency.
Quite often, technological innovation has less to do with the creation of new systems than it does with rethinking the way existing systems are used and configured. That’s what we’ve done in this case – and will continue to do in our ongoing effort to give designers and engineers the tools they need to develop increasingly creative solutions that meet client needs and expectations in a changing world.
Jeff Freeman is founder and owner of Fluid Logics, an Upland, Calif.-based manufacturer of electrolytic chlorine generators. In addition to being a licensed Certified Pool Operator (CPO), he has been inventing, guiding agency regulatory policy and lecturing before countless watershaping organizations for more than 30 years – while also participating as a designer, consultant and builder of many noteworthy watershapes. Recently, Freeman has dedicated his career to the advancement of water purification and sanitization technology. He can be reached at jeff@fluidlogics.net.