Limestone Solutions

With the intensification of global climate change, reducing atmospheric and oceanic carbon dioxide levels has become a unifying pursuit that has given rise to innovative approaches. Including one based entirely on dissolved limestone to combat acidification.
By Lauren Stack
Ever since the beginning of the Industrial Revolution, humans have been pumping increasing levels of CO2 into our atmosphere and waters, causing levels to rise to unprecedented concentrations in the industrial era – ultimately raising global temperatures, as well as causing ocean acidification and temperature rise.
For these reasons, methods aimed at removing CO2 are being developed with increasing urgency, and capital investment. The method described here is but one of numerous measures that are in development. In this case, the process relies on the simple concept of pumping massive amounts of limestone into rivers and other bodies of water. It’s an example of managing chemical balance on a potentially global scale.
It’s basic chemistry, not unlike managing pH in a swimming pool or spa, but just on a much, much larger scale. The limestone method being developed by an innovative firm, CarbonRun, is in concept elegantly simple. It’s based on the natural chemical reaction between limestone and CO2 in water, ultimately helping to buffer pH levels, combat ocean acidification and the effects of acid rain, all while storing CO2 in stable bicarbonate compounds. While the “limestone method” offers an elegant solution based on chemistry fundamentals, challenges of scale and implementation remain daunting. Nonetheless, the case for limestone capture remains compelling.
“Our work here has the potential to drive meaningful change on a broader scale,” said Shannon Sterling, CarbonRun founder, chief scientific officer and chief operating officer. “By restoring the natural chemistry of rivers, we are reviving their role in the global carbon cycle, which is crucial in the fight against climate change.”
Here’s a look at how the technology works, how it has been applied to mitigate environmental degradation, the challenges of scaling the method, and the potential long-term impacts on ocean acidification and CO2 removal from the atmosphere.
THE LIMESTONE APPROACH
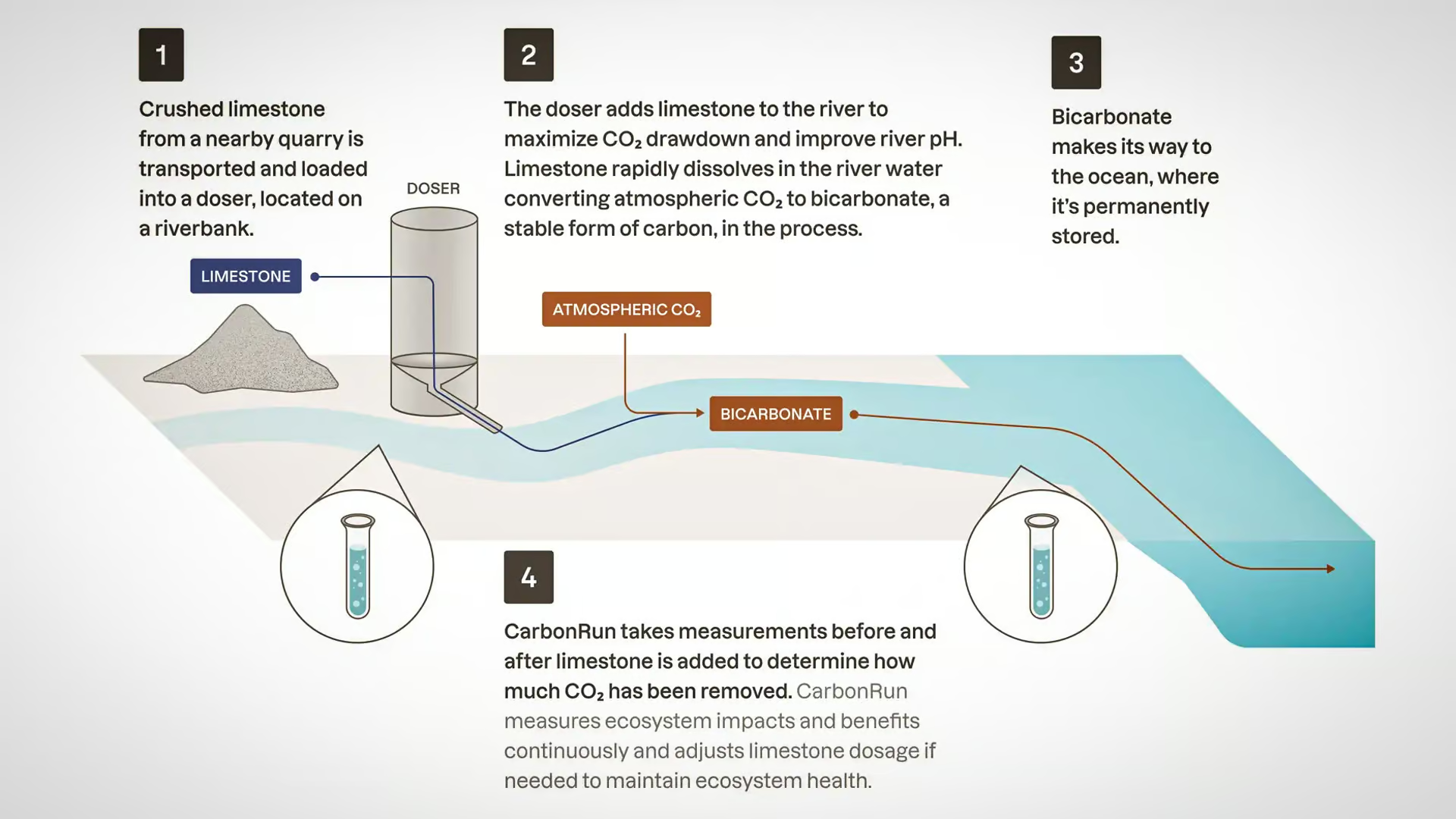
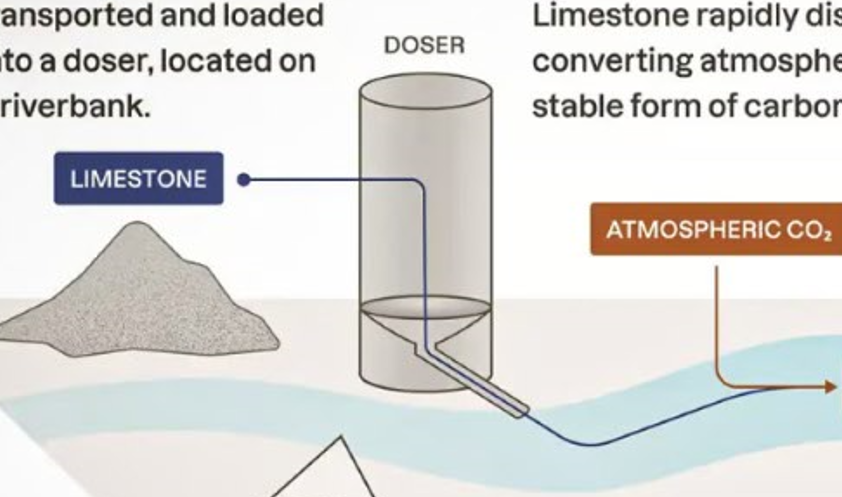
The core concept involves adding pulverized limestone that is dissolved into the waters of rivers, lakes, and coastal waters to increase the alkalinity of water and facilitate a reaction that removes CO2 from both the air and the water. When limestone dissolves in water, it reacts with carbon dioxide to form bicarbonate (HCO₃⁻), a stable and safe compound.
The reaction can be summarized as:
CaCO₃ (limestone) + CO₂ + H₂O → Ca²⁺ + 2HCO₃
In this basic process the limestone (CaCO₃) neutralizes dissolved CO2 in water by converting it into bicarbonate. Bicarbonate is far less reactive than dissolved CO2 and serves as the long-term storage form of carbon in the oceans, where it remains stable for centuries or longer.
The process also increases the water’s pH, ultimately counteracting ocean acidification and stabilizing aquatic ecosystems that are threatened by low pH levels.
The simplicity of the chemical reaction, the abundance of limestone, and its relatively low cost make this an appealing strategy for large-scale carbon sequestration and acidification reduction.
ACID RAIN MITIGATIONS
Before the introduction of the method as a solution for climate change, limestone was successfully used to counter the effects of acid rain in freshwater ecosystems. In the 20th century, industrial activities such as coal burning released large amounts of sulfur dioxide (SO₂) and nitrogen oxides (NOₓ) into the atmosphere, leading to acid rain. Acid rain lowered the pH of rivers and lakes, damaging fish populations, aquatic plants, and entire riparian systems.
Limestone neutralization became a key tool in environmental management, with powdered limestone being introduced into affected rivers and lakes to raise pH levels and mitigate the damage. In North America and Europe, this method was implemented widely, restoring aquatic life in acidified water bodies by buffering the acidity and restoring ecosystems’ balance.
Building on these successful applications, the company seeks to apply the same principle to tackle the broader issue of CO2 emissions, which cause both ocean acidification and atmospheric warming.
From a systems and components perspective, the method integrates proven processes and technologies that work together. The system operates at different scales, from localized river installations to broader coastal deployment, depending on the specific environment and goals of the project.
Limestone was chosen because it is abundant, inexpensive, and highly effective at neutralizing acidity in water. Pulverization and dispersion mechanisms are used to maximize the reaction efficiency between limestone and CO2. The limestone is finely pulverized into a powder. This increases the surface area available for the reaction, maximizing the reaction efficiencies between limestone and CO2.
Pulverized limestone is then dispersed into rivers, lakes, or coastal waters using various methods such as pumps, automated spreaders, or mechanical feeders. In river systems, the flow naturally helps distribute the limestone downstream, while in coastal or stagnant waters, additional mixing technologies may be employed to ensure even distribution.
CARBON SINKS
Monitoring and controlling pH levels to ensure that the water is not over-alkalized is a critical system component. pH sensors, which are deployed in the treated water bodies, measure acidity levels before and after limestone application.
The data collected from pH sensors help regulate how much limestone is needed and when more limestone should be added. This prevents over-application, which could disturb the ecosystem; and, it ensures that the CO2 capture process is optimized without negative side effects.
Understanding how water flows, and how dissolved chemicals are transported, are key to the success of the entire process. Water flow models are used to predict how limestone and its reaction products (bicarbonate) will disperse throughout the water system.
These models account for river currents, tidal movements, and ocean mixing to optimize limestone dispersion and ensure that the bicarbonate formed stays within the ecosystem for long-term carbon storage. In some cases, physical barriers or flow regulators may be introduced to control the spread of bicarbonate within certain areas.
MAKING IT WORK
The system begins with identifying suitable water bodies where limestone application can yield the greatest benefits. Key factors include the current pH of the water, its CO2 levels, and whether it flows into larger marine ecosystems that are affected by acidification.
Once the site is selected, the limestone is finely ground and dispersed into the water using pumps or mechanical spreaders. In river systems, the limestone is usually applied at key points where water flow can carry it downstream for maximum coverage.
Continuous monitoring of the water’s pH and bicarbonate levels is carried out to ensure the process is working as intended. If necessary, additional limestone is added to maintain the correct balance and ensure long-term effectiveness.
Once bicarbonate is formed, it remains stable in the water, effectively sequestering CO2 over long periods. This process continues passively, with ongoing monitoring to ensure that the water chemistry remains balanced and beneficial to local ecosystems.
The need for solutions such as this method becomes even more apparent when considering the long-term effects of ocean acidification and warming.
Ocean acidification weakens coral skeletons, threatening the long-term survival of coral reefs, which support 25% of all marine life. Acidified oceans disrupt the development of marine organisms that rely on calcium carbonate to form their shells and skeletons, such as mollusks, crustaceans, and some plankton species. As acidification affects foundational species, the broader marine food web is at risk, potentially leading to reduced biodiversity and affecting fisheries, which millions of people rely on for food.
By stabilizing bicarbonate in ocean waters, this approach offers what proponents believe is a promising method to counteract ocean acidification and capture CO2 long-term.
CHALLENGES OF SCALE
Despite the clear benefits of the limestone approach, scaling it up to have a global impact comes with significant challenges. These include logistical, environmental, and technological concerns, as well as the need for long-term monitoring. Logistical challenges include:
- Sourcing and Transport: Limestone is abundant, but the process of mining, crushing, and transporting the necessary quantities to key waterways on a large scale could be costly and energy-intensive. Delivering limestone to remote river systems and oceanic regions also adds complexity and expense to the operation.
- Strategic Placement: Identifying which river systems and coastal areas would benefit most from limestone addition requires careful hydrological and ecological assessments. Water currents, tidal flows, and other environmental factors will determine how effectively the limestone disperses and how long it stays active.
Of course, there are numerous environmental concerns. Proponents of the method don’t want to create additional impact issues while seeking to address climate change. Those concerns include:
- Ecosystem Disruption: While limestone has been used to neutralize acidity, its application at large scales raise questions about potential unintended consequences for ecosystems. Sudden changes in water chemistry, particularly in delicate river systems, could affect local biodiversity, especially if the limestone deposits alter habitats or food webs.
- Over-Alkalization: While increasing alkalinity in acidified waters can be beneficial, there is a risk of over-alkalization if limestone is applied in excess. Careful monitoring of water pH is crucial to avoid harming aquatic life or disrupting natural chemical balances.
CALLIBRATING EXPECTATIONS
The effectiveness of the limestone method depends on maintaining optimal conditions over long periods. This requires continuous monitoring of pH levels, bicarbonate concentrations, and CO2 levels in both water and air. Long-term success depends on refining the method based on real-world data, which could be difficult to collect consistently at large-scale.
The limestone method may work better in certain environments than others. Factors such as water temperature, salinity, and existing chemical composition will affect how effectively CO2 is sequestered as bicarbonate. Customizing the approach to fit different regions is a challenge that requires significant research.
While the limestone method offers promising potential for reducing CO2 levels and combating ocean acidification, realistic expectations for its large-scale deployment should be tempered by the challenges outlined above.
Initial large-scale deployments will likely focus on regional applications in areas that are already severely affected by acidification or CO2 pollution. River systems that feed directly into high-value coastal ecosystems, such as coral reefs or fish habitats, could be prioritized.
Limestone applications will not provide an immediate fix for climate change or ocean acidification. It will require years of continued effort to see measurable improvements in ocean pH and atmospheric CO2 levels. However, the method’s stability, with bicarbonate potentially storing carbon for centuries, suggests that the long-term impact could be significant.
ONE OF MANY
Rather than a stand-alone solution, the limestone method should be viewed as part of a broader portfolio of climate solutions. It works well in conjunction with reforestation, carbon capture and storage, and renewable energy deployment, contributing to an overall reduction in CO2 emissions and environmental damage.
The limestone method offers a unique and scientifically sound approach to addressing two of the greatest environmental challenges of our time: ocean acidification and atmospheric CO2 levels. While there are significant challenges to scaling the method, including logistical, environmental, and technological hurdles, the potential benefits make it a worthy addition to the suite of global climate solutions.
By converting harmful CO2 into stable bicarbonate compounds, the method not only provides long-term carbon storage but also helps buffer ecosystems against the damaging effects of acidification. With careful management and ongoing research, this innovative approach holds significant promise for the future of carbon sequestration and marine conservation.
Lauren Stack is vice president of business development for Watershape University. She holds a bachelor’s degree in chemistry from the University of Pittsburgh, graduating summa cum laude.
Photos by Eric Herman.