Testing Know-How
|
Even watershapers who don’t perform daily tests of water quality in the systems they design and/or build will benefit from being familiar with the various methods available for water analysis, says Michael Gardner of Taylor Technologies. Such knowledge, he notes, helps in starting up new systems, formulating chemical-treatment regimens, calibrating automatic controllers or simply educating those who’ll care for the watershapes you create. |
Watershapers pour their artistic vision, engineering skill, architectural know-how and construction expertise into projects that clients expect to enjoy for years to come. Lots of factors determine the longevity and successful operation of those watershapes, but one long-term-satisfaction point stays consistently near the top: water quality.
Those of you who have been following Jeff Freeman’s water-chemistry series in WaterShapes already know that water must be balanced to prevent damage to surfaces and equipment as well as sanitized to protect the health of people who come in contact with it. In addition, water in living systems such as ponds and streams must be managed to prevent harm to aquatic plants and animals.
So, how can you ensure acceptable levels of water quality? Simply stated, you must test it. Indeed, regular testing is the cornerstone of any effective treatment program.
In this primer on test methods, we’ll explore the analytical methods commonly associated with water-testing kits – colorimetric, titrimetric, turbidimetric and electronic – so you can choose the best one for your needs or make sound recommendations to clients and service providers.
COLORFUL SOLUTIONS
In a colorimetric (that is, a color-matching) test, chemicals called reagents are added to a water sample, where they react with the analyte of interest (that is, free or total chlorine, copper, ammonia or some other component of the water). The interaction of reagents and analyte produces a color proportional to the analyte’s concentration. This color is then compared to a set of color standards until a match is found.
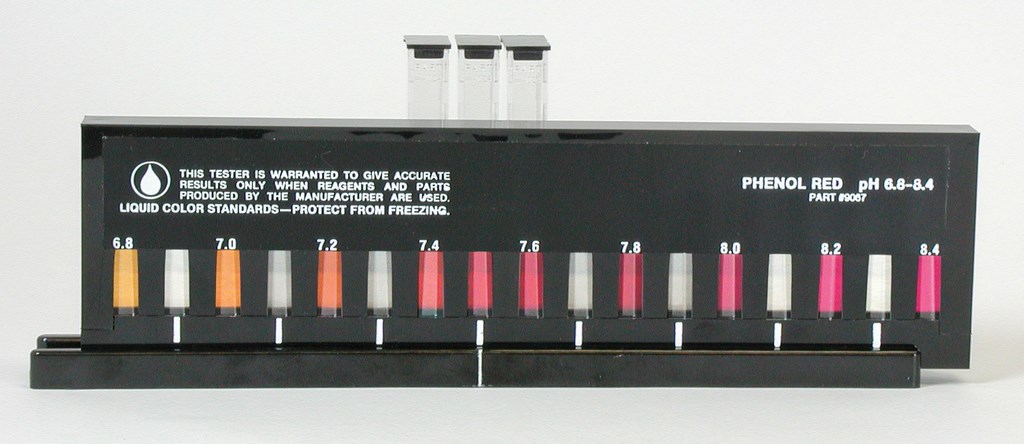
| Figure 1: Many test kits use color comparators. Although the one pictured here uses printed color standards, others may use colored-plastic or liquid standards. |
There are several types of color comparators on the market, including printed, liquid and colored-plastic standards. All three types require you to differentiate among colors – often hues in the same range, such as the shades of yellow used in many chlorine tests or the shades of pink in pH tests (Figure 1) – to determine chemical concentration.
|
TESTING TIP: * Whenever testing outdoors, remove your sunglasses before making a reading because the tinted lenses will interfere with color perception. |
In these comparative methods, you must follow instructions carefully to obtain accurate results. Some test suppliers, for example, may ask you to hold the color comparator at eye level with the sun off to the side (that is, not shining directly through the comparator nor directly onto the faceplate) when testing outdoors. When testing indoors, by contrast, it may be necessary to use a special lamp – neither incandescent nor fluorescent – to simulate daylight and ensure a proper color match.
Before selecting a colorimetric test, it’s important to know the likelihood of encountering color or turbidity in the sampled water, as either one of those factors will interfere with color matching. If, for example, you’re planning to analyze pH in green or colored pond water or chlorine in cloudy, winterized pool water, you must either filter the sample before testing or choose a test system that compensates for the interference (Figure 2).
| Figure 2: Comparators such as this one will compensate for color and turbidity in a water sample. |
And regardless of the type of color-matching system you choose, you must be certain to select a kit with color standards that reflect your target range of concentrations.
IN PRINT
Despite the fact that neither printed colors nor colored plastic can exactly match the characteristics of an aqueous sample, such comparators are often used in measuring sanitizer levels, water balance parameters and the metals content of watershapes.
|
TESTING TIP: * If you are among the estimated six to eight percent of the population who lives with red/green deficiencies in your color vision (mainly males), some colorimetric tests will be problematic. Be aware of your limitations and look for alternatives. The color standards used with DPD chlorine tests are all shades of pink, for example. If you have trouble differentiating among the hues, use an FAS-DPD titration test instead – or get a colorimeter and have it read the sample for you. |
There are, for example, many liquid test kits that ask you to dose water samples and then compare the color that develops to printed standards or blocks of tinted plastic corresponding to a range of concentrations for the analyte of interest.
Test strips also require matching colors against a printed standard (Figure 3 below). Strips are now used for a wide range of water tests in both sanitized and living systems and are particularly common these days in testing of ponds and lakes, where parameters such as pH, alkalinity, hardness, ammonia, nitrites and nitrates must be monitored to protect the health of plants and fish.
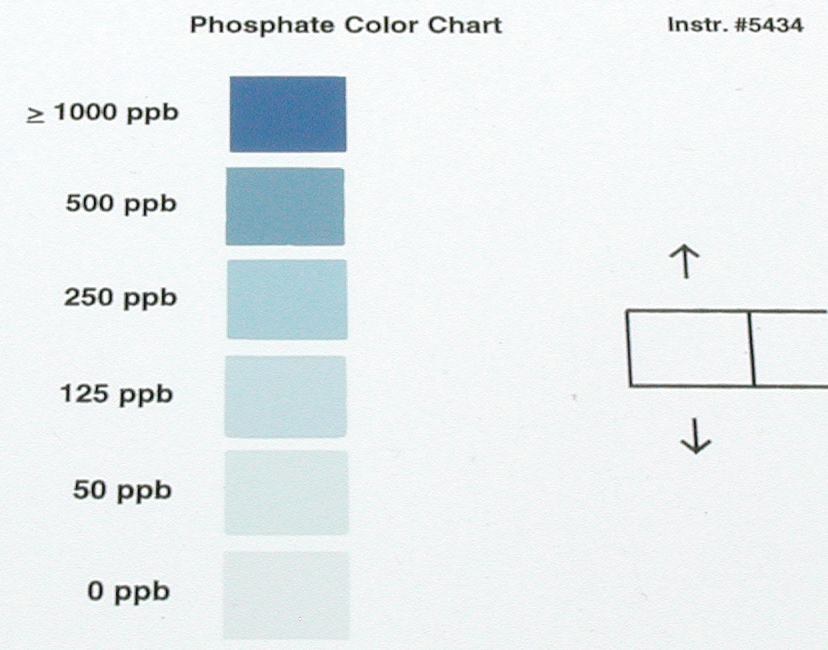
| Figure 3: Simple color-card tests are available for monitoring many parameters such as pH, phosphate and copper to name a few. |
When using these test strips, you simply “dip and read,” matching the color that develops on each pad against color standards that are usually affixed to the container (Figure 4 below).
To ensure accuracy, you must be sure to follow the manufacturer’s instructions regarding immersion time and whether to swish, swirl or dunk the strip to expose the reagent system to the water sample. You also need to follow instructions about how much time you must let elapse before taking a reading.
| Figure 4: Be sure to hold test strips level when comparing colors to prevent water from running between the reagent pads and contaminating the tests. |
Test strips are generally recommended for quick checks of watershapes when there’s a high probability that the tested water-quality parameters will be within the expected ranges. Suppose, for example, that you’re using a typical test strip to determine total alkalinity in a pool: Its container has printed standards for 0, 40, 80, 120, 180 and 240 parts per million and you get a reading somewhere between 40 and 80 ppm. How much sodium bicarbonate should you add to increase the alkalinity to your target of 100 ppm?
The simple answer is that you don’t know exactly because the test strip doesn’t operate with the level of resolution needed to calculate a dosage. In this case, it would be better to use a drop-count test, which measures total alkalinity in 10-ppm increments and lets you more accurately determine what you need to do to treat the water properly.
LIQUID LOGIC
To avoid problems associated with the consistency and accuracy of printed standards, many test kits employ liquid-to-liquid color matching.
|
TESTING TIP: * With printed standards, important quality distinctions include how close the printed colors actually come to target colors, whether the manufacturer consistently achieves the same colors from press run to press run, and how the standards hold up under constant use in wet environments. Your best bet: Use kits with standards printed on laminated or waterproof paper. |
In these tests, you add reagents to a water sample that subsequently develops a color proportional to the concentration of the analyte of interest. You then compare this color to multiple liquid standards housed in a handheld comparator, looking for the closest match (Figure 5 below). A typical iron-content comparator, for example, might include liquid standards at 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5 and 2.0 ppm in hues that range from light to dark purple.
| Figure 5: Liquid-to-liquid color comparators provide extremely accurate color matches and are available in a wide variety of parameters and test ranges. |
Liquid-to-liquid comparators are extremely easy to use, portable and available in the ranges most often encountered in watershapes. If you exercise reasonable care in handling these tools – that is, don’t drop them, leave them out in the sun or let them freeze – you will enjoy many years of service from a modest investment.
|
TESTING TIP: * Look for reliable quality: The best liquid standards are guaranteed not to fade over the lifetime of the comparator. |
DROP BY DROP
With some water-quality parameters, the method for testing will take you back to your high-school chemistry class and a process known as titration.
Alkalinity, hardness, bromine, chloride and chlorine levels, for example, can all be monitored with this simple procedure. First, you collect a sample of the water to be analyzed and carefully measure it (according to instructions) into a graduated sample tube or flask. Next, you add a special color-changing indicator chemical.
The indicator will change color at a specific point in the reaction; this is called the endpoint and is the moment in the analysis when the test reading should be taken. Finally, a reagent of known concentration, referred to as the titrant, is added incrementally until a permanent color change takes place.
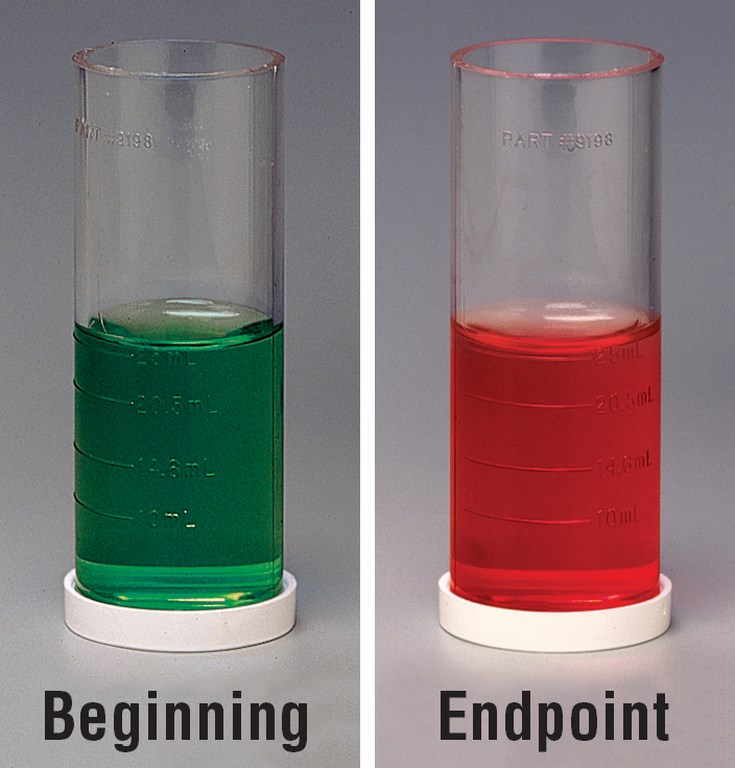
| Figure 6: In a titration-based test, such as the total alkalinity analysis shown here, a chemical indicator changes color at a specific point in the reaction, signaling that a reading should be taken. |
In a total alkalinity test, for example, you will see the treated sample turn from green to a mix of green and red and, finally, to all red at the endpoint (Figure 6). The concentration of the analyte of interest is calculated based on how much titrant was added. (Suppliers recommend conducting these tests against a white background so you can clearly see the progress of the reaction and the endpoint’s color change.)
|
TESTING TIP: * In performing titrations, swirl the tube after adding each drop to mix the reagent thoroughly into the sample. When in doubt about whether or not a permanent color change has occurred, add one more drop of titrant: If the color does not change any more, do not count that last drop. |
Generally, test kits designed for use in the field feature a specific type of titration called a drop-count titration or drop test. Titrant is dispensed from a dropper bottle or pipet into the treated water sample until the endpoint is reached. These tests are quite popular because they are portable, require minimal expertise, can be performed in a minute or two even by inexperienced analysts, offer a degree of accuracy sufficient for most applications (depending upon the quality of the dropper’s tip) and are quite economical.
In addition, these titration systems have few components, require no calibration and need little upkeep. Some manufacturers offer systems that use highly accurate microburets for dispensing the titrant; there are also “reverse titration” kits in which the sample is added to the titrant until the endpoint is reached.
SEEKING CLARITY
The testing of cloudy water is a special sort of water analysis. The cloudiness (or turbidity) is caused by solids suspended in the water.
|
TESTING TIP: * In titration testing, be sure to hold the dropper bottle or pipet straight up and down when dispensing reagent to ensure proper drop size. If you notice a diminishing drop size, you need to remove the static that’s causing the problem by wiping around the tip of the dropper with a clean, damp paper towel. (To prevent contamination, wet the towel with a few drops of the reagent.) |
Turbid water’s cloudiness is caused by suspended solids. Outdoors, these solids would likely include algae and sediments. The naturally occurring turbidity of a pond or stream can be used to gauge its general water quality; a Secchi disk may be used for this purpose on site, or a portable turbidimeter can be used on site or off.
In some tests, turbidity is deliberately created in a water sample as a means of measuring the level of a specific analyte. With pools and spas, for instance, the water is made cloudy in the process of testing for cyanuric acid, the chemical used to slow ultraviolet degradation of chlorine.
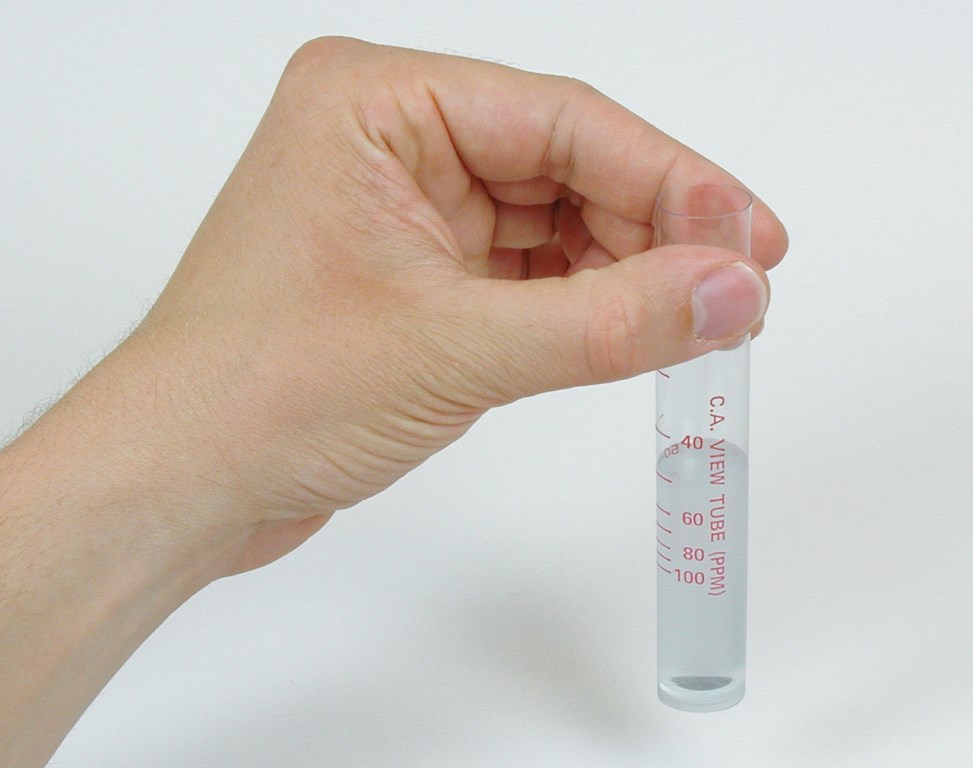
Many watershapers are familiar with the cyanuric acid test in which there’s a black dot on the bottom of a test cell (Figure 7). You pour reacted sample water containing a whitish precipitate into it. When you can no longer see the dot, you take the reading from calibration marks on the side of the test cell.
| Figure 7: The calibration marks on this test cell indicate the concentration of cyanuric acid in parts per million. The reading is made when a black dot on the cell’s bottom is obscured by cloudiness. |
Such a test operates on the same principle as the Secchi disk: The degree to which a marker in the test cell is obscured by the cloudiness of the treated water sample correlates to the concentration of the analyte of interest.
ELECTRONIC ASSISTANCE
As might be expected, many water-quality tests can now be performed with electronic systems that take much of the subjectivity and approximation out of the equation. When properly maintained and routinely calibrated, these microprocessor-based instruments offer a high level of accuracy in field testing.
|
TESTING TIP: * In performing titrations (or any of these tests, for that matter) avoid direct contact with reagents. Many contain harmful substances. In addition, soil and oil transferred from your hands will leave them unfit for testing. |
Handheld meters, for example, can be used to measure parameters such as pH, temperature, conductivity, resistivity, total dissolved solids (TDS), oxidation-reduction potential (ORP) and many other parameters of interest to those who test and treat the water in watershapes.
Some instruments measure just one parameter, while others measure many. The best are built to withstand the demands of field work and feature waterproof, dustproof, and chemical-resistant housings, ergonometric designs, intuitive operation, pre-programmed methods for multiple analytes, automatic “housekeeping,” easy interfaces with a personal computer or printer and the potential for a certain amount of customization. These meters enable even those with minimal knowledge of water chemistry to be successful water analysts.
|
Testing Techniques In addition to the tips offered throughout the accompanying text, there are some basic points to follow in any good testing regimen. • Before testing, be sure your hands and work area are as clean and dry as possible. Also, keep a notepad, logbook, or PDA nearby to record test results. • Familiarize yourself with test instructions before beginning a test – especially when running a test that’s new to you – and follow the steps exactly. Note any special considerations stated in the instructions, such as required waiting times, safety warnings and conversion factors for test results. • Before beginning, make sure you have all the reagents and labware needed to perform the test – and verify that all components are in good condition. • Review test instructions and field manuals carefully for information on potential test interferences and how to avoid them. • For meaningful test results, take a water sample representative of conditions in the whole system. • Before gathering a sample, always rinse the test cell with the water to be tested to prevent contamination from any residue. This is especially important when using the same test cell to analyze multiple parameters or when using equipment at multiple testing locations. • When gathering a sample for off-site analysis (at a pool/spa or pond supply shop, for example), fill the container to overflowing and cap it so no air is present. • With on-site analyses, test immediately after sampling, since some values (halogen sanitizers in particular) can change within minutes. • After testing, flush out the test cell with demineralized water and wipe equipment down with a clean, dry cloth. • To prevent contamination of reagents, don’t interchange container caps and always replace them securely. • For the same reason, never cap a sample container with your finger while testing. • If exchanging one manufacturer’s reagents with another’s, be sure they’re of equivalent strength. • To keep reagents fresh, store them out of direct sunlight at 36 to 85 degrees Fahrenheit (2 to 29 degress Celsius) and away from treatment chemicals. Also avoid extreme temperature fluctuations. • Refer to the manufacturer’s product literature for information on the useful life of reagents. — M.G. |
Electronic controllers go one step further by continuously analyzing water chemistry and activating mechanical or chemical water-treatment systems as needed to keep various levels within target ranges.
While once limited mainly to laboratory environments, colorimeters and spectrophotometers are beginning to see greater use in field testing in their portable forms. The technologies employed in these units make them far more sensitive to light and color than the human eye, and they analyze samples in the same way each and every time, which leads to repeatable results.
Simply put, these devices are both accurate and precise. The basic premise is this: Light passes through a prepared water sample and reaches a detector that measures the amount transmitted through or absorbed by the treated sample. (Light of different wavelengths may be used, depending upon the parameter being analyzed.) This measurement of transmitted or absorbed light is then converted to a reading of concentration by software in the meter – a great thing when you need to monitor a parameter at ultra-low levels.
Electronic instruments, however, are not yet a replacement for all other methods. Their price can be prohibitive, for example, in light of the fact that the wet chemistry tests discussed above can do the job much less expensively. Further, they require more care and can go out of commission unexpectedly (although some suppliers offer service loaners for a fee). Still, no one can afford to ignore the benefits of the systems instrument manufacturers have been making available for water analysis.
MAKING CHOICES
This survey of available techniques and technologies is by no means exhaustive and should serve only to introduce basic concepts and offer you some guidance in the sort of testing associated with starting up and maintaining watershapes of various types.
There are tests for just about every detail of water composition for both sanitized and living systems, and knowing your way around them – at least to the extent of knowing what features can be tested and what the results imply – is good, commonsense practice in a realm where client satisfaction is everyone’s goal.
It’s all about understanding the options: If you know what’s available and why and how it is used, you’re better positioned to choose or make sensible recommendations among the many possibilities and find the test that’s best suited to a given application. As you make your decisions, the goal should always be ensuring accurate test results, defining an effective treatment program and, ultimately, figuring out the best way to keep your clients happy.
Michael Gardner is communications coordinator at Taylor Technologies, the Sparks, Md.-based manufacturer of water-testing products. He earned his bachelor’s degree in English from Penn State University in 1997 and a master’s in publication design from the University of Baltimore in 2002. He has contributed articles about water chemistry and testing to numerous trade publications; edits newsletters for several of the firm’s major markets, including the pool/spa industry; and produces catalogs, fliers, and other technically oriented materials for water analysts.