Indoor Water & Air Quality (part I)


Indoor pools, known as natatoriums, are notorious for bad air quality. These facilities are unique in the world of design, construction and maintenance because they contain open bodies of water. In this two-part series, Eric Knight describes the basic framework for maintaining quality water and air in these special aquatic spaces.
By Eric Knight
It’s no secret that many indoor pool facilities (natatoriums) are often plagued with poor indoor air quality (IAQ). Those problems exist because swimming pools introduce factors not found in other types of commercial rooms (think gymnasiums, auditoriums, cafeterias, classrooms, and offices).
Increased moisture loads, relative humidity, vapor pressure, high temperatures and chloramine pollution, all stem from an indoor swimming pool. All these factors conspire to make indoor pools notoriously difficult to design and maintain. On top of that, there are relatively few indoor pools in the world. That means relatively few professional engineers have ever designed them. Even fewer have designed them well.
And to be fair, creating a pleasant, manageable and sustainable indoor pool environment is a tall order. It involves a spectrum of interrelated systems and multiple professional disciplines. These disciplines include air handling, environmental control, water quality, overall facility design, program management and maintenance routines.
Rarely do these specialists speak to each other…and if they do, they speak different languages.
Chloramine Consulting exists today to be the bilingual bridge between air and water designers. We provide design assistance to mechanical engineers and also evaluate troubled facilities in-person. Every natatorium is unique and there is no single cure-all to air and water quality problems. Although the recipe is a little different each time, knowing the basics is a good start. The fundamental physics are always the same.
CHICKEN OR THE EGG?
In my opinion, it’s unfair to aquatic facility operators to say that all these problems can be prevented by just having good water chemistry. That is NOT true. Even “perfect” water chemistry will have disinfection byproducts (DBPs) because they are a necessary byproduct of chlorine oxidizing nitrogen compounds that are introduced by bathers.1
In truth, natatorium airflow and HVAC design are equally–if not more–important. But for the purposes of this article, we will start with water quality. In Part 2, we’ll talk about air.
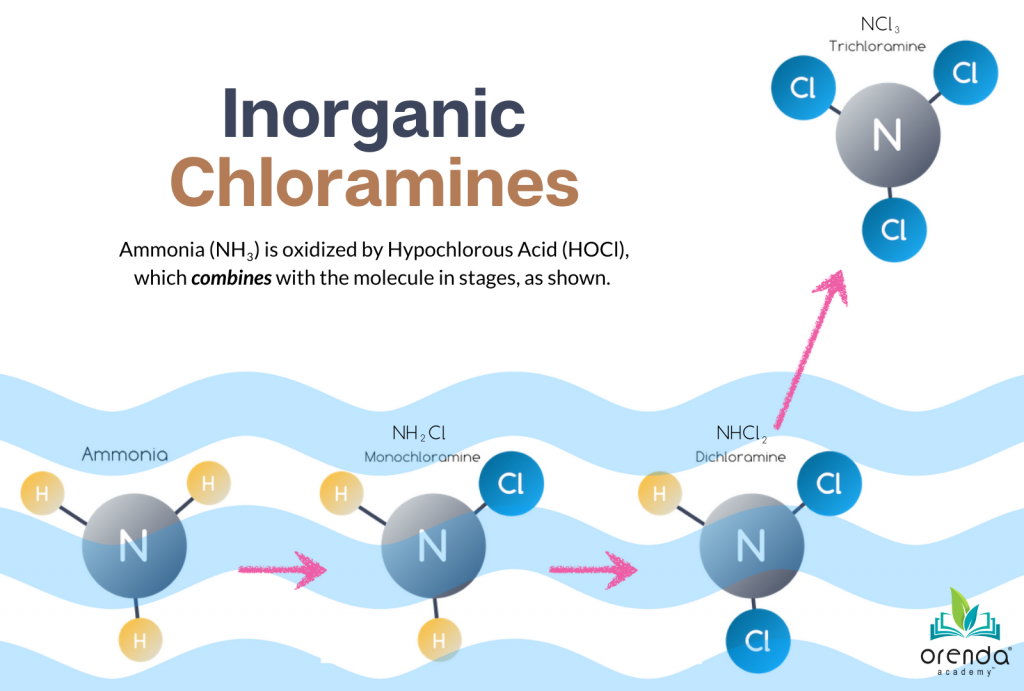
The process of chlorinating swimming pool water generates chloramines and a host of other potentially harmful disinfection byproducts (DBPs). These are the chemical culprits that compromise water quality and find their way into the air. In the industry, we generally refer to all DBPs as “chloramines”, though that’s not technically accurate. Actual chloramines (mono-, di-, and trichloramines) are inorganic chloramines. They are created when chlorine combines with ammonia.
Without going through all of the dozens of subsequent chemical reactions, basically chlorine combines with ammonia to create monochloramine.
HOCl + NH3 → NH2Cl + H2O
Hypochlorous acid + ammonia → monochloramine + water
From there, more chlorine combines with monochloramine and other nitrogen compounds to produce other byproducts. Many of these are harmful irritants that pollute natatoriums, and those that off-gas are the root of indoor air quality problems.
Almost 90% of ammonia in the water will eventually off-gas as Nitrogen gas, N2. About 10% will become nitrate ions, NO3–, and less than 1% will off-gas as nitrogen trichloride, NCl3 (aka trichloramine).2
Just that <1% of ammonia becoming trichloramine is still enough to cause bather discomfort and health issues like ‘lifeguard lung’. I can confirm this because I personally developed asthma and lifeguard lung during my swimming career. It’s miserable.
BATHER HYGIENE
So how do ammonia and other nitrogen compounds get in water in the first place? Usually it’s from bathers and chemicals that contain ammonia.1 When a pool has combined chlorine, identifying the source of nitrogen is the first step in addressing the problem.
The fact is, chloramine may not be the pool operator’s fault. The operator is limited by the design and quality of the circulation and filtration systems. Yes, shoddy service routines can be part of the problem, but we’ve found that’s usually not the case. Unlike residential pools, indoor commercial pools are usually used to capacity by people who may not make helpful hygiene decisions.
Specifically, operators have no control whether or not swimmers shower before they enter the water. Showering before swimming is nice in theory, but its impractical because people don’t like getting cold before they jump in the water.3 So that’s not going to be an easy cultural change to make unless hot showers on the pool deck become more commonplace.
Swimmers also pee in the pool, and for valid reasons. Sure, it’s disgusting, but the common belief in swimming is that “chlorine kills urine”. In reality, urine is sterile, and chlorine oxidizes it. Urine is rich in urea, which oxidizes into ammonia and various chloramine byproducts.
Not only is it cold to get out of the water during practice to pee, there isn’t much time to do so. On top of that, competition swimsuits are difficult to remove for bathroom breaks–especially female suits.
No one likes getting cold, especially when it means losing your warm-up during a workout. But this could theoretically be alleviated by coaches implementing planned bathroom breaks. But 50 to 60 wet swimmers trying to share limited bathroom facilities is impractical. There is simply no easy way around the issue of peeing in the pool.
All of this is why robust water treatment and maintenance is the primary way to satisfy the water-quality side of the indoor pool equation.
When you consider the sheer numbers of people using more popular facilities, it’s easy to see how that spells trouble for the even the most diligent operators. If the circulation, filtration, and chemical treatment systems are inadequate, it can be impossible to maintain quality water and a pleasant indoor environment. Given the conditions in most natatoriums, the formation of some level of DBPs is inevitable.
Once DBPs form and enter the air through evaporation and off-gassing from the water’s surface, water chemistry becomes a thing of the past. At that point, airborne contaminants must be removed by air-handling systems. Therefore, water chemistry management can only go so far in mitigating air quality issues. Nonetheless, reducing the formation of chloramines and other DBPs to the greatest extent possible is important.
THE BREAKING POINT
This all means that pool operators must destroy the nitrogen – either chemically or mechanically. But how?
Chemically reducing combined chlorine
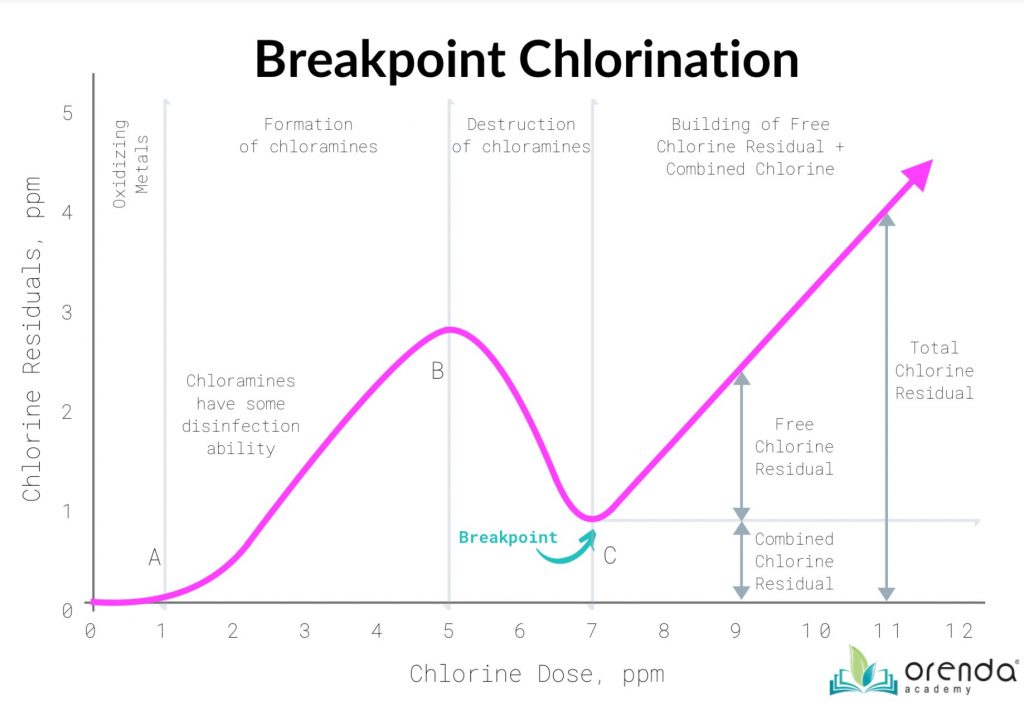
Chemically speaking, superchlorination is far and away the most widely used practice for reducing combined chlorine. Superchlorination is adding an excess of free chlorine (the industry standard practice is 10-times the level of combined chlorine, though technically that’s more than necessary). This surge of oxidizing power should destroy the chloramines – and the rest of the oxidant demand–and allow for a free chlorine residual to build. The point where a free chlorine residual begins to build is known as “the breakpoint”, and that’s where the term breakpoint chlorination comes from.
While it is sometimes necessary, superchlorination is not the ideal way to handle the oxidant demand. If you properly supplement chlorine, normal chlorination itself should be able to stay above the breakpoint. The key is to reduce the oxidant demand on chlorine, even if those oxidants are not nitrogen compounds. The vast majority of oxidants in water are non-living organics, which can be broken down and removed by enzymes.
Enzymes like CV-600 and CV-700 have made dramatic improvements in indoor air quality, despite them not being able to address nitrogen itself. By removing non-living organics, free chlorine has less demand on it, which allows for more rapid and thorough oxidation of nitrogen compounds.
You can also use a non-chlorine shock like potassium monopersulfate. While it will not kill germs or oxidize combined chlorine directly, potassium monopersulfate is a powerful oxidizer that can destroy nitrogen precursors in the water.
Mechanically reducing combined chlorine
If you’re going to have an indoor pool, it should have a secondary disinfection system on it. Let’s break these secondary systems into two categories: UV, and secondary oxidizers like Ozone, AOP and HDO.
Ultraviolet (UV) systems can destroy monochloramines in water. Both low and medium-pressure UV can destroy monochloramines. Medium pressure can also destroy dichloramine and aqueous trichloramine. UV is not an oxidizer, however, so it cannot destroy nitrogen precursors. It is also a point-of-contact system, so it can only affect the water that flows through it.
Oxidizers like Ozone and Advanced Oxidation Process (AOP) are also point-of-contact systems, but they destroy just about everything. Any nitrogen compounds or combined chlorine that ozone or AOP’s hydroxyl radicals touch will be destroyed in fractions of a second.
There is also a relatively new type of secondary system, and that is Hyper-dissolved oxygen (HDO). HDO is not a powerful oxidizer like ozone or AOP, but it boosts chlorine and enzyme performance, and it absolutely enhances water quality.
SUMMARY
While many factors come into play for water quality, supplementing chlorine against the oxidant demand is the the most important thing you can do as a pool operator. We recommend enzymes and a secondary system. By combining treatment technologies, you can create powerful synergies in which chlorine has less work to do, and therefore forms less DBPs.
The bottom line: when you remove the oxidant demand in the form of nitrogen-containing compounds, both water and air quality will inevitably improve.
Next time, we’ll discuss the air side of this issue.
Eric Knight is a former competitive swimmer and American Record Holder. He is devoted to fixing indoor air quality (IAQ) problems in natatoriums and is currently the VP of Business Development for Orenda Technologies.
___________________________________________________
1 Nitrogen compounds are also introduced to swimming pools in other ways. In outdoor pools, the main culprits are ammonia-based algaecides, fertilizers and decaying organic material like leaves, soils and grass clippings. But for indoor pools, the main source of nitrogen would be ammonia-based deck cleaners and other cleaning products. There are also chloramines in drinking water in some parts of the country. If you have combined chlorine, the first priority is to identify where the nitrogen is coming from. Then address that source as best you can.
2 Source: Renowned water chemistry expert Richard A. Falk
3 Evaporative cooling is real. Just ask any swimmer whose teeth chatter while standing on the pool deck soaking wet. It’s usually over 82ºF on deck and swimmers are shivering thanks to water evaporating off their skin. Showering before swimming is cold and uncomfortable, and therefore it has been difficult to convince swimmers to do so.
Opening photo by crystal51 | Shutterstock